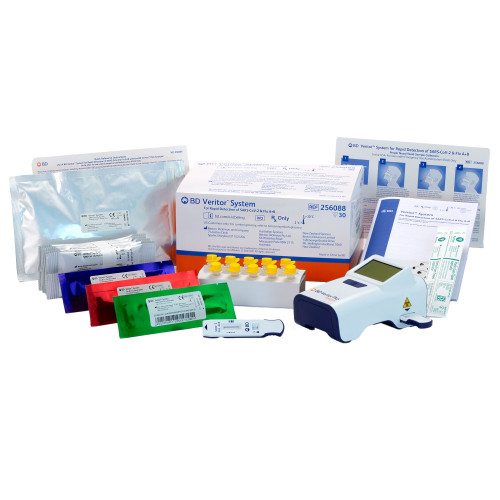
- The BD Veritor System for Rapid Detection of SARS-CoV-2 and Flu A+B is intended for use in point of care settings by trained healthcare professionals or other users specifically instructed in the use of BD Veritor Systems and proper infection control procedures.
- Time to result: 15 minutes, test device can be read at 15 minutes but no later than 20 minutes.
- This product has been authorized only for the detection of proteins from SARS-CoV-2, influenza A and influenza B, not for any other viruses or pathogens.
- BD Triplex assay requires Veritor™ System firmware version 5.5 or later; firmware version info is displayed on screen when turning on the analyzer or on the orange sticker if it is attached to the analyzer.
- This test is authorized for use at the Point of Care (POC), i.e., in-patient care settings operating under a CLIA Certificate of Waiver, Certificate of Compliance, or Certificate of Accreditation certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA), 42 U.S.C. §263a, that meet the requirements to perform moderate, high or waived complexity tests.
- SKU:
- 256088--BD
- UPC:
- 382902560883
BD 256088 - Respiratory Test Kit BD Veritor™ System SARS-CoV-2 / Influenza A + B 30 Tests CLIA Waived
- Item Number:
- 256088
- Manufacturer:
- BD
 Estimated Shipping 3-5 Business Days*
Estimated Shipping 3-5 Business Days*