- Nucleocapsid antigen from SARS-CoV-2 may be detected within the first 5 days of onset of symptoms.
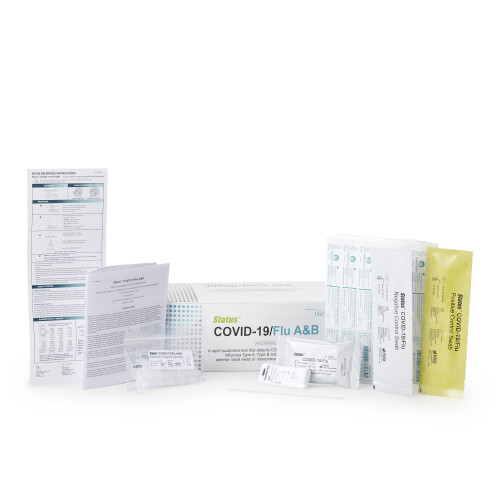
- Contents include: 25 test devices; 25 extraction reagent capsules; 25 sterile swabs; positive control swab; negative control swab; and package insert quick reference instructions.
- Results in 15 minutes
- Status Covid-19 / Flu A and B test has been granted emergency use authorization (EUA) by the FDA for use at the point-of-care setting.
- Kit contents include 25 test devices; 25 extraction reagent capsules; 25 sterile swabs; positive control swab; negative control swab; package insert and quick reference instruction.
- SKU:
- 33225--LIF
Lifescan Inc 33225 - Respiratory Test Kit Status COVID-19 / Flu A and B 25 Tests
- Item Number:
- 33225
- Manufacturer:
- Lifescan Inc
 Estimated Shipping 3-5 Business Days*
Estimated Shipping 3-5 Business Days*